
Hi everyone, this is Min,P.E. and I am writing this post on "Types of Electrolyzers within Hydrogen Economics".
There are three main types of electrolyzers used in the hydrogen economy: alkaline, proton exchange membrane (PEM), and solid oxide.
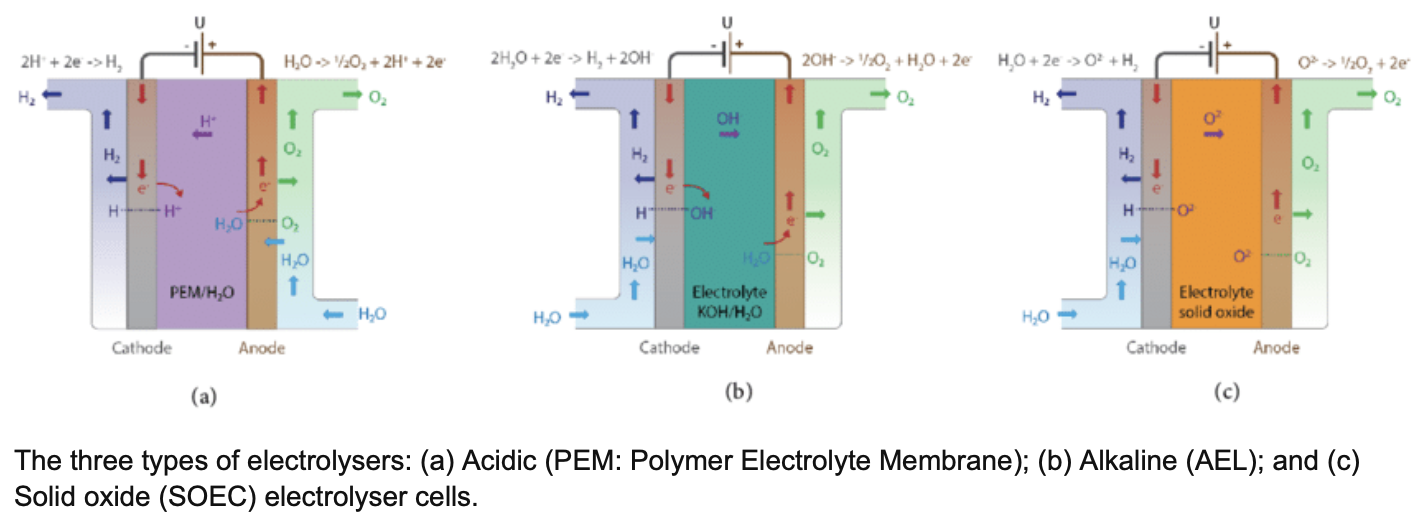
- Alkaline electrolyzers are the oldest and most mature technology, and they are currently the most widely used type of electrolyzer. Alkaline electrolyzers use a liquid alkaline electrolyte, such as potassium hydroxide or sodium hydroxide, to conduct ions between the electrodes. Alkaline electrolyzers are relatively inexpensive and efficient, but they operate at a lower electrical efficiency than other types of electrolyzers.
- PEM electrolyzers are a newer technology, but they have quickly become popular due to their high electrical efficiency and fast response time. PEM electrolyzers use a solid polymer electrolyte membrane to conduct ions between the electrodes. PEM electrolyzers are more expensive than alkaline electrolyzers, but they are also more efficient and can be used for a wider range of applications.
- Solid oxide electrolyzers (SOECs) are the most efficient type of electrolyzer, but they are also the most expensive and require high temperatures to operate. SOECs use a solid oxide ceramic electrolyte to conduct ions between the electrodes. SOECs are still under development, but they have the potential to be the most cost-effective type of electrolyzer for large-scale hydrogen production.
The type of electrolyzer that is best suited for a particular application depends on a number of factors, including the scale of the project, the operating environment, and the cost of electricity.
For example, alkaline electrolyzers are a good choice for small-scale hydrogen production applications, while PEM electrolyzers are a good choice for medium- to large-scale applications. SOECs are still under development, but they have the potential to be the best choice for large-scale hydrogen production applications in the future.
Here is a table that summarizes the key characteristics of the three main types of electrolyzers:
| Alkaline | Potassium hydroxide or sodium hydroxide | 60-90 | 60-70 |
| PEM | Solid polymer electrolyte membrane | 60-80 | 70-80 |
| SOEC | Solid oxide ceramic electrolyte | 700-800 | 80-90 |
The hydrogen economy is a vision for a future energy system in which hydrogen is used as a clean and sustainable energy carrier. Electrolyzers play a key role in the hydrogen economy by producing hydrogen from water using renewable energy sources. As the hydrogen economy develops, electrolyzers will become increasingly important for producing clean and sustainable hydrogen.
This is the end of this post and i will also let you know further with continued posting with hydrogen economy.
'003. 에너지(Power & Energy) Note' 카테고리의 다른 글
| EMS와 SCADA의 차이점 그리고 그 특징은? (17) | 2023.10.16 |
|---|---|
| The overview of wind turbine concepts and power output equation (3) | 2023.10.16 |
| Transmission-Line Differential equations (Telegrapher's equations) (6) | 2023.10.11 |
| 수소 경제에 대한 개념, 특징 그리고 전망 (10) | 2023.10.10 |
| [23.9.4 기준] 국내 송변전 설비 현황 및 전력수급 현황 분석 자료 (19) | 2023.09.04 |